
Our unique selling proposition is the production of specific and/or customised products within a short time.
Althena Team, alongside its partners (Customers, Suppliers and Consultants), is a firm believer of “knowledge management”. This belief is based on our direct experience, where we have made the best possible use of the knowledge acquired, starting from patents up to internal operating processes and information obtained from our customers.
In a world where business is characterised by continuous technological acceleration, the base of “corporate knowledge”, that is our “expertise”, is the actual competitive advantage we can provide to our customers.
This precious company resource, the “Althena Medical’s expertise”, is protected (patents), cultivated and shared with all our employees and partners.
We therefore produce products that provide tangible benefits to customers and users and of a Quality exceeding current standards.
The development stages of the devices, from design to production, are all characterised by our “knowledge management”, meaning our “Expertise”.
All company departments, Engineering, Quality Control, Quality Assurance, Research and Development, Production, are perfectly integrated with each other. They collaborate from the earliest stages of device development up to production. Collaboration and continuous exchange of views are crucial for the success of each project and for meeting deadlines and maintaining the highest company standards.
Conception and Design
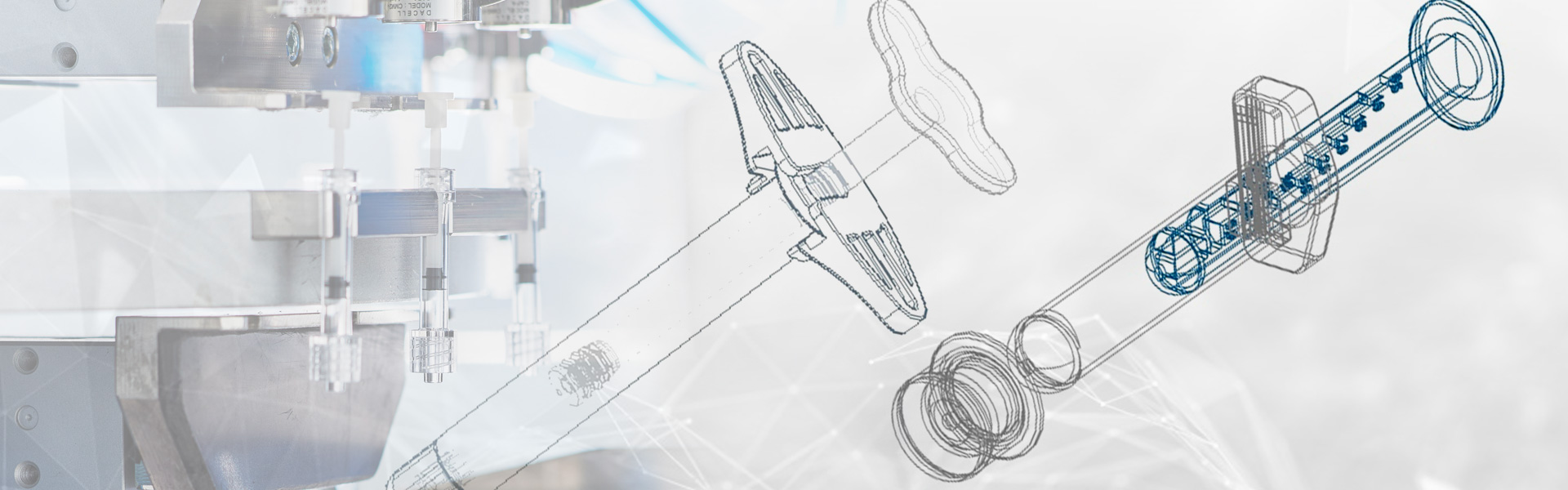
Our first step is listening to the customer to provide them with the best possible “feasibility analysis” (request one as well, now and with no constraints, from here).
After receiving an initial agreement from the customer on the feasibility analysis, we propose two or three technical solutions. Our experience, our sector and trans-sectoral knowledge allow us to always find the technical solution that best suits the preliminary analysis shared with the customer. We often employ concepts and solutions deriving from sectors neighbouring our own whilst apparently distant from us.
Once the design has been chosen, it is shared with the customer, in order to proceed with the creation of 3D prototype moulds that optimise the proposed design. The first laboratory tests, usability tests, etc then begin. From the results of these tests, new 3D printing prototypes are created, until the optimal design is obtained after several steps, which will then be produced via injection moulding.
Research, development
and Prototyping

Thanks to the latest generation tools such as 4K technology, reverse engineering and specially trained personnel, the first prototypes of the device made through “injection moulding” are carefully and thoroughly measured to the hundredth. This is in order to verify the compliance of the injection moulding prototypes with the initial project. Furthermore, the material withdrawal is calculated, the presence of any defects and/or “burrs” and critical aspects such as the formation of internal air bubbles, potentially dangerous for the resistance of the component, are checked. The pilot moulds are optimised within one or two steps at most.
Once the withdrawals and manufacturing defects have been verified, we then move on to the creation of the final moulds. We work closely with the mould makers, transferring them the results of the measurements on the first prototypes and sharing their technical choices. Synergy and comparison are essential at this stage.
This results in:
- a high quality of each piece produced;
- fast production times;
- better quality/price ratio.
Supply chain
The entire supply chain is fully integrated with the company and it is local. There is a perfect combination and integration throughout mould making. In fact, those who make our moulds and those who directly print the pieces share our Values and our “Expertise”.
The supply chain is short and local so as not to be affected by international crises.
Engineering Department
Our modus operandi requires the “Research and Development” department to constantly collaborate with engineering.
All our products, both those commissioned by Customers and those developed with our Brand, are made with a view to production in a “clean room” and therefore accompanied by the creation of assembly tools and automatic systems. This way, the entire manufacturing process of our devices is monitored and supervised with a view to marketing carried out in the shortest time possible. From conception to production, the technicians of the engineering department design both the tools for device assembly and the machines, always in a 4.0 perspective, interfacing with suppliers for the creation of both pilot and final moulds. They also actively participate in the testing of the machines and equipment they have designed.
All maintenance, both predictive, routine and special, is the responsibility of the Engineering Department. It thus acquires increasingly more experience becoming more and more efficient. The Engineering Department also trains machine operators. Therefore, it is involved from the testing stage of the developed devices, up to the start and continuation of production, following the entire life cycle of the devices.
Patents
We know how important it is to patent and protect ideas that truly benefit both our devices and those of our customers. For this reason, the Research and Development Department has been trained from a patent point of view to search for patented solutions that may interfere with our own, and to find technical solutions easier to safeguard with a patent application. Therefore, the risk of interfering with third party patents or others copying our solutions is very low.
Furthermore, internal staff are supported by external professionals who have been following us for years and have in-depth knowledge of our sector, our products and those of our competitors.
Thanks to these synergies and our internal knowledge (Expertise), we are able to safeguard our ideas and those of our customers, significantly reducing the risks when launching the devices on the market.
Criticality analysis
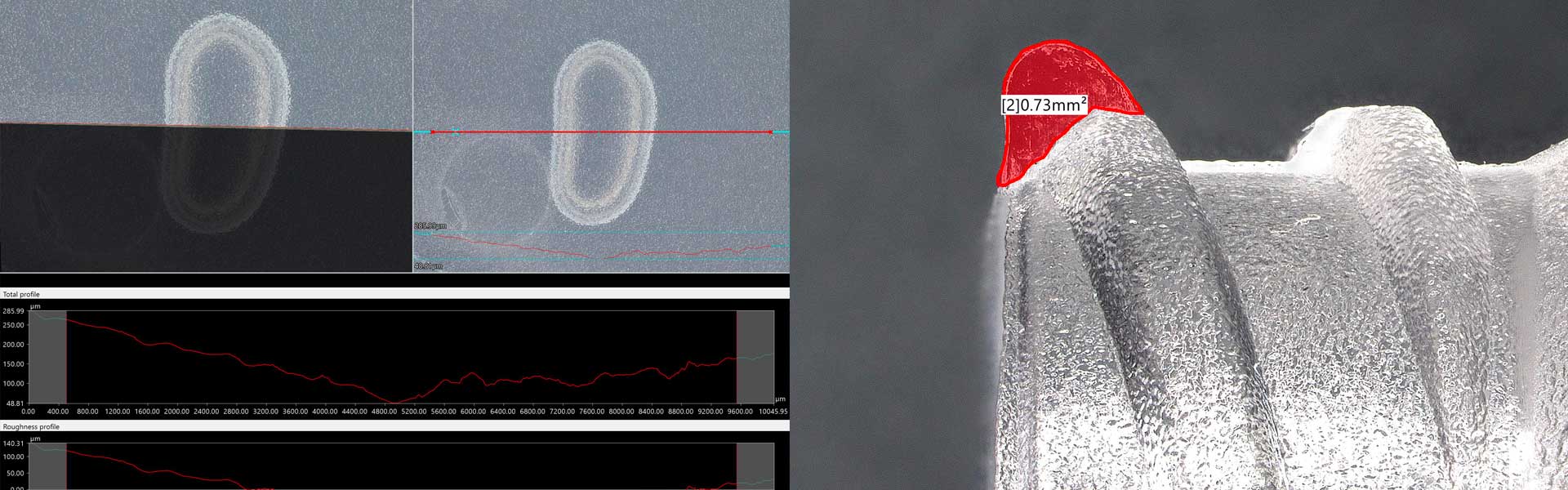
Another Company Plus: the strict “controls” on the performance of the devices.
Before being placed on the market, our devices are subjected to various tests, be in compliance with the regulations in force in order to verify achievement of the performance and specific requests of the customer. The tests are carried out both internally and at external laboratories.
For example:
in the case of our Sycare, the pre-fillable syringe in COP, several tests were performed at external institutes, such as:
- ageing;
- airtightness with methylene blue;
- smoothness of the plunger in the piston;
- test in autoclave;
- machinability test (emptying and filling syringes by using automatic machines);
- strength test, to prevent cracks and breakages.
In the case of our Dosecare, oral liquids dispensing device, it was tested by 200 testers who were then asked specific questions to verify all the characteristics of the devices. The following have been performed:
- usability tests, using 200 testers at a specialised institute;
- smoothness test of the piston in the barrel;
- barrel filling test;
- volumetric measurement tests of liquids drawn into the barrel.
The airtightness test carried out on the Sycare confirmed an excellent result with respect to other competitors: the airtightness of Sycare guarantees the liquid inside it for up to 3 years or more from its filling.
Usability test at specialised institutes
Usability tests are especially useful in order to fulfil the satisfaction of our customers and Prospects, who also care about the aesthetic and handling aspects of the product.
The results of our tests can be requested, without obligation, by filling out our “contact form“.
Quality system

Quality control
Our quality system is ISO 13485:2016 certified. However, our internal controls go far beyond the obligations of our system.
The quality control of our devices is carried out by specialised and specifically trained personnel. Various measurements of different parameters are carried out periodically in order to verify and prevent defects. The devices are analysed under a microscope utilising 4K technology. This allows us to eliminate any problems at the source..
All defects are catalogued and counted and the data are reported in special tables. This allows us to monitor their progress over time so as to predict the their causes.
Spot checks are performed on the incoming materials and only the suitable ones move on to the assembly stage.
We are subject to periodic on-site audits by all our customers. Our production standards are pharmaceutical-grade. Our manufacturing standards are pharmaceutical grade.
The quality of our products is a priority for us. Each device we produce represents us and tells our story.
Quality assurance
The QA department personnel is constantly updated on new regulations. They attend courses and participate in seminars and webinars. They work closely with all other departments. A characteristic of this department, like the others, is that it is integrated into all company processes. In fact, its interaction with all company functions is crucial for the success of each individual project.
QA drafts all documents for the CE marking of the devices, drafts the operating instructions, prepares for both internal audits and those at external suppliers. It also trains the internal personnel for quality checks.
Manufacture

If the Design, Research and Development sector is the Heart of Althena, production is the real Engine.
We have developed a Know-how that allows us to produce both small and large batches with a high quality content and quickly.
Internal production can be manual, automatic or collaborative.
Our automatic assembly machines are often designed by our internal Engineering Department which also follows the development stages of our products.
All machines were built with a 4.0 perspective, and collaborative robots and cobots were extensively used.
Periodic, predictive, routine, and special maintenance, under the supervision of the Engineering Department, guarantees the continuity of production.
The personnel is flexible and interchangeable in order to always ensure continuity.
The supply chain is short and local so as not to be affected by international crises.
Marketing
For Althena, marketing means first of all creating stable and long-lasting relationships with customers and prospects, which are reflected in our company values: transparency, reliability, orientation towards tangible results.
Therefore, we make ourselves known through by publishing articles relating to experiments carried out by us and/or on current issues.
We are present at major international events such as CphI and Pharmapack. We create informative videos and publish the opinions of our prospects and customers. We try to create a network with companies and prospects who believe in continuous innovation and in the constant search for quality and oriented towards achieving results.
